Sanitizers are one of frequently purchased products world-wide especially due to the current COVID-19 pandemic. The efficacy of the hand hygiene product determines its activity against bacteria, yeasts, and coated viruses.
Among the available products, alcohol-based sanitizers are in trend due to its active action against germs such as bacteria, fungi and viruses. Alcohol is treated as a good antiseptic and does not show toxic effect on human skin however, continuous use may cause irritation and dryness. Different concentrations of alcohol are available in the market. Although a higher concentration of alcohol acts effectively against germs, the best efficacy can be achieved by using ethanol (60-85%), isopropanol (60-80%), and n-propanol (60-80%) based sanitizers. As per literature, 95% ethanol has the highest action against naked viruses. Moreover, the synergistic action could be seen by mixing a different combination of alcohols. As proof, propanol and isopropanol are presently available as good disinfectants in the market.
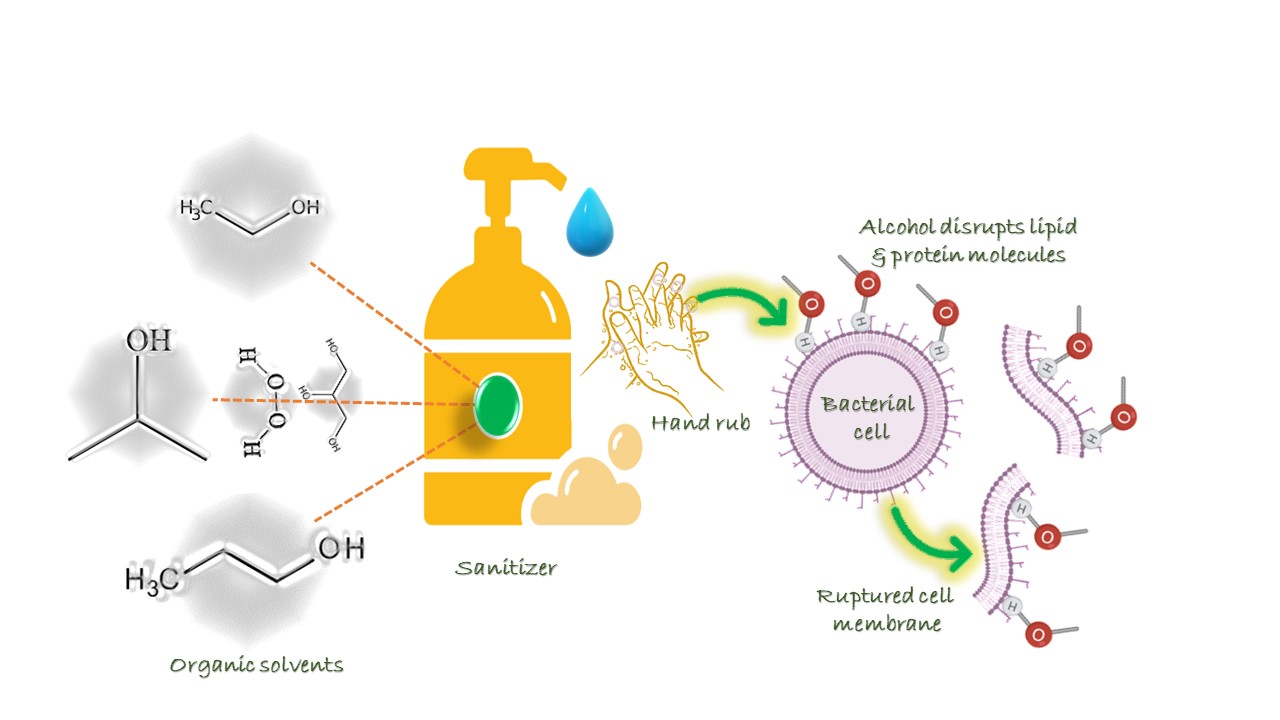
Why alcohol is so important in sanitizer?
A bacterial membrane entails basic compounds such as phospholipids and lipopolysaccharides and their interactions are alleviated by Mg2+ and Ca2+ ions. When ionized, disinfecting molecules are absorbed or repelled by electrical charges at the initial contact and absorption stage, leading to cell membrane damage and the electrolyte leakage which can activate cell death pathways.
Alcohol is a combination of organic molecules such as carbon, oxygen, and hydrogen. Alcohol clears most of the disease-causing pathogens by breaking the protein-lipid and dissolving their membranes. The bacterial membrane contains hydrophobic or lipophilic tails and a hydrophilic head phosphate group. Sanitizers act at lipophilic ends to break the cell membranes by rupturing its structure and denaturing the proteins. The novel SARS-CoV-2 virus is surrounded by a ‘fatty’ membrane layer, which can also be disrupted by using alcohol-based sanitizers. In particular, when the concentration of alcohol exceeds 60%, it has high efficiency to kill certain bacteria and viruses. In addition to alcohol, limited concentrations of hydrogen peroxide are added to avoid the growth of microbes and glycerol or caprylyl glycol or isopropyl myristate is added to moisturize the skin and prevents dermatitis. Moreover, water acts as a catalyst in sanitizer, which helps to improve penetration and leads to cell membrane rupture. Besides, fragrances are also used to give a pleasant odour. When sanitizer is rubbed on hands, ethanol evaporates and leaving behind soothing compounds.
Not only alcohol-based sanitizers, even less concentrated i.e., 0.3% of benzalkonium chloride or triclosan based sanitizers also kill germs. However, the effectiveness is lower compared to alcohol-based sanitizers. So, alcohol and its percentage are very important in sanitizer preparation and purchase.
Written by: Kumara B N, DST-SERB-JRF, Yenepoya Research Centre, Yenepoya (Deemed to be University), Mangalore-575018, Karnataka, India.

Artwork: Kumara B N
Edited by: Dr. Pratigya Subba